
论文题目:Engineering Co/MnO heterointerface inside porous graphitic carbon for boosting the low-temperature CO2methanation
论文作者:Wengang Cui, Xinying Zhuang, Yanting Li, Hongbo Zhang, Jingjing Dai, Lei Zhou, Zhenpeng Hu, Tongliang Hu*
Abstract:
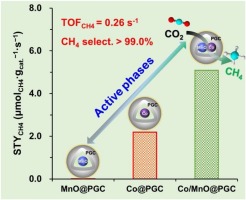
Direct conversion of CO2 into CH4 provides an eco-friendly way of mitigating CO2 emissions and reducing the demand for fossil fuels, but this process was kinetically hindered under low temperature because of the high stability of CO2 molecule. The search for efficient low-cost catalysts capable of converting CO2 into CH4 with a high space-time yield (STYCH4) at low temperature is becoming more desirable. Here we report a highly efficient, stable and core-shell catalyst with abundant Co/MnO heterointerface inside porous graphitic carbon (Co/MnO@PGC), which can be easily obtained by one-step calcination of a bimetal-organic framework (CoMn-MOF-74). The combination of various the nanoscale characterizations and theoretical modeling ascertain that the in situ generated Co/MnO heterostructured nanoparticles (NPs) not only create many metal defects and oxygen vacancies, but also enhance the Co-MnO interaction at Co/MnO heterointerface, which promote CO2 adsorption and facilitate CO2 activation. The resulting Co/MnO@PGC is able to convert CO2 to CH4 at temperature even as low as 160 degrees C with >99% selectivity and exceptional high STYCH4 of 0.14 mu mol(CH4).s(-1).g(cat.)(-1), surpassing by far the most active Co catalysts reported up to now under the identical condition. Most astonishingly, at a higher pressure (30 bar), the STYCH4 can reach up to 5.60 mu mol(CH4).s(-1).g(cat.)(-1) at 160 degrees C, which is comparable to the optimal level of Ru-based catalyst, and simultaneously sets a new benchmark for the Co-based methanation catalyst. On the basis of catalytic studies and in situ FTIR spectroscopy of CO2 methanation experiments, the active sites responsible for this superior performance for Co/MnO@PGC can be associated with a synergy between Co degrees and MnO at the Co/MnO heterointerface, where H-2 is efficiently dissociated on the Co degrees and the strong adsorption and activation of CO2 taking place on the adjacent MnO. This work provides a promising way for the design of advanced CO2 methanation catalysts.