
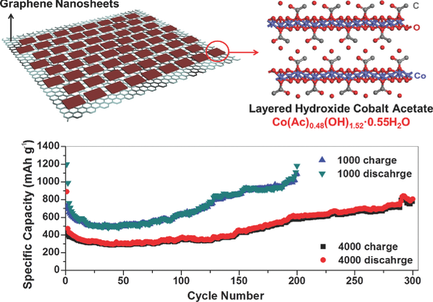
The dramatically increasing demand of high-energy lithium-ion batteries (LIBs) urgently requires advanced substitution for graphite-based anodes. Herein, inspired from the extra capacity of lithium storage in solid-electrolyte interface (SEI) films, layered hydroxide cobalt acetates (LHCA, Co(Ac)0.48(OH)1.52·0.55H2O) are introduced as novel and high-efficiency anode materials. Furthermore, ultrathin LHCA nanoplates are face-to-face anchored on the surface of graphene nanosheets (GNS) through a facile solvothermal method to improve the electronic transport and avoid agglomeration during repeated cycles. Profiting from the parallel structure, LHCA//GNS nanosheets exhibit extraordinary long-term and high-rate performance. At the current densities of 1000 and 4000 mA g−1, the reversible capacities maintain ≈1050 mAh g−1 after 200 cycles and ≈780 mAh g−1 after 300 cycles, respectively, much higher than the theoretical value of LHCA according to the conversion mechanism. Fourier transform infrared spectroscopy confirms the conversion from acetate to acetaldehyde after lithiation. A reasonable mechanism is proposed to elucidate the lithium storage behaviors referring to the electrocatalytic conversion of OH groups with Co nanocatalysts. This work can help further understand the contribution of SEI components (especially LiOH and LiAc) to lithium storage. It is envisaged that layered transition metal hydroxides can be used as advanced materials for energy storage devices.