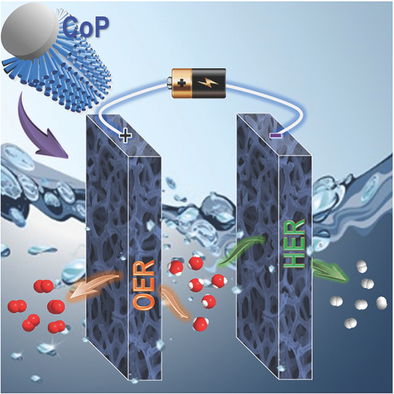
Water splitting for the production of hydrogen and oxygen is an appealing solution to advance many sustainable and renewable energy conversion and storage systems, while the key fact depends on the innovative exploration regarding the design of efficient electrocatalysts. Reported herein is the growth of CoP mesoporous nanorod arrays on conductive Ni foam through an electrodeposition strategy. The resulting material of well-defined mesoporosity and a high specific surface area (148 m2 g−1) can be directly employed as a bifunctional and flexible working electrode for both hydrogen and oxygen evolution reactions, showing superior activities as compared with noble metal benchmarks and state-of-the-art transition-metal-based catalysts. This is intimately related to the unique nanorod array electrode configuration, leading to excellent electric interconnection and improved mass transport. A further step is taken toward an alkaline electrolyzer that can achieve a current density of 10 mA cm−2 at a voltage around 1.62 V over a long-term operation, better than the combination of Pt and IrO2. This development is suggested to be readily extended to obtain other electrocatalysis systems for scale-up water-splitting technology.